Millions of people are confined to wheelchairs due to spinal cord injury, diabetes, multiple sclerosis, infirmity and other conditions. For wheelchair users, the loss of feeling, lack of mobility, and poor circulation can cause large, difficult to heal, pressure sores that can extend to the bone. Once the skin breaks down, pressure sores take time to heal and the skin may break down again and again.
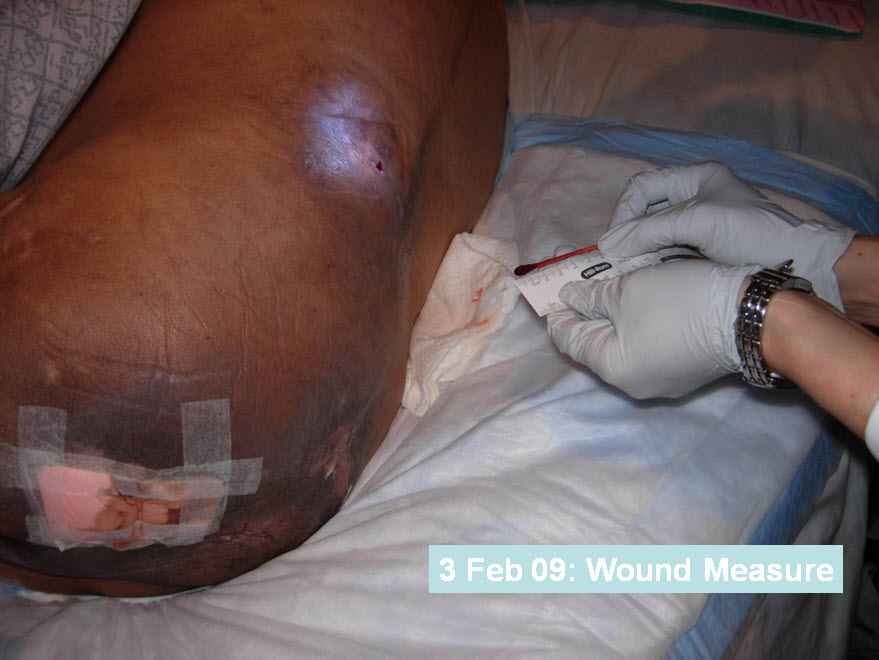
Patients are often confined to bed—sometimes for years—in an effort to heal the wounds. Such confinement is devastating for the patient in terms of mobility, social contact, and work and on family members and caregivers. Pressure sores are a leading cause of death for wheelchair users.
The Generic Total Contact Seat (GTCS) offers hope to people confined to wheelchairs because pressure sores can be healed while the patient sits in a wheelchair and is mobile rather than confined to bed. Patients can participate in family and social activities, recreate, and work while healing.
The GTCS is individually sized to the patient’s anatomy for maximum effectiveness. The seat redistributes weight to anatomical areas that can better handle pressure: the outer wall of the pelvic ring and the posterior thigh. The air support surface system provides high air loss therapy for interface pressures below capillary pressure to prevent skin breakdown while the inflating and deflating bladders allow blood circulation to all skin areas by shifting pressure points.
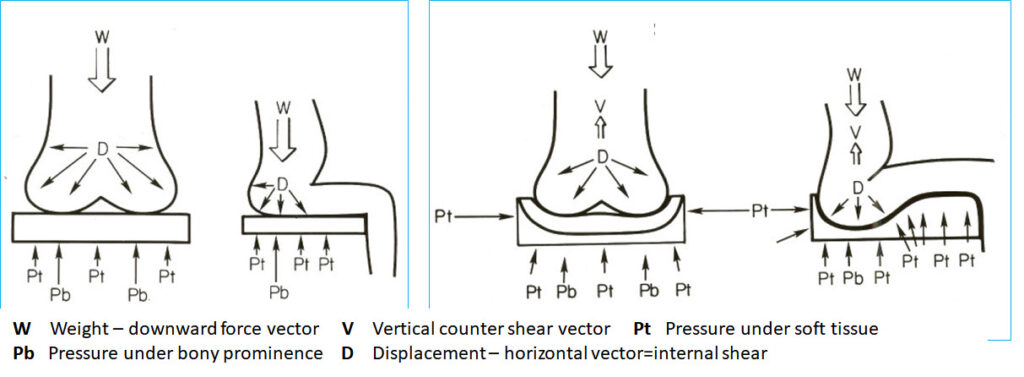
Implementing the valve and pressure system required technical expertise. Sandia National Laboratories developed the GTCS air bladders and the associated electronics under a $1.5 million Cooperative Research and Development Agreement with Numotech. Sandia scientists drew on their expertise in battery technology, miniaturization, and energy conservation to create a reliable, effective and economical device. The air bladders inflate and deflate using a patented sequencing valve arrangement. The piston-driven biomechanical action of the GTCS™ compresses buttocks tissues, encourages circulation and prevents tissue death.

Clinical studies validated the effectiveness of the GTCS and proved that the device increased blood supply, stimulated tissue growth, reduced treatment time by one-third, and prevented new pressure sores. The FDA cleared the GTCS as a Class II medical device.
The seat is portable; it is easily inserted and removed from a wheelchair. The seat fits standard wheelchairs size 14-20 and includes an 8-hour rechargeable battery pack.
The GTCS was selected by Popular Science magazine from among thousands of new products and technology developments to win the magazine’s 2001 “Best of What’s New” Award for Medical Technology.


The GTCS priced at about $1,500 offers significant cost savings compared to air loss beds costing $???.
GTCS Testimonial

Publications
A wheelchair cushion designed to redistribute sites of sitting pressure
Mark J Rosenthal, MD; Robert M Felton, PhD; Anne E Nastasi, MD; Bruce D Naliboff, PhD; Judith Harker, PhD; Joseph H Navach, MD (2003) Healing of advanced pressure ulcers by a generic total contact seat: 2 randomized comparisons with low air loss bed treatments1. Journal of Physical Medicine and Rehabilitation 84(12):1733-1742.
Mark J. Rosenthal, MD; Robert M. Felton, PhD; Dale L. Hileman, BS; Martin Lee, PhD; Joseph H. Navach, MD (1996) A wheelchair cushion designed to redistribute sites of sitting pressure. Journal of Physical Medicine and Rehabilitation 77(3): 278:282.

