Numotech is a medical device company focused on wound care with a product pipeline of market-ready, FDA-cleared disruptive medical technologies that revolutionize wound care treatment.
All products are clinically transformative and low-cost innovations that meet currently unmet wound treatment needs. They are industry game changers that meaningfully meet real-time wound care challenges and set new standards of care. Numobag® Kit, the flagship product, heals non-healing wounds in record time at low cost and without patient use restrictions.
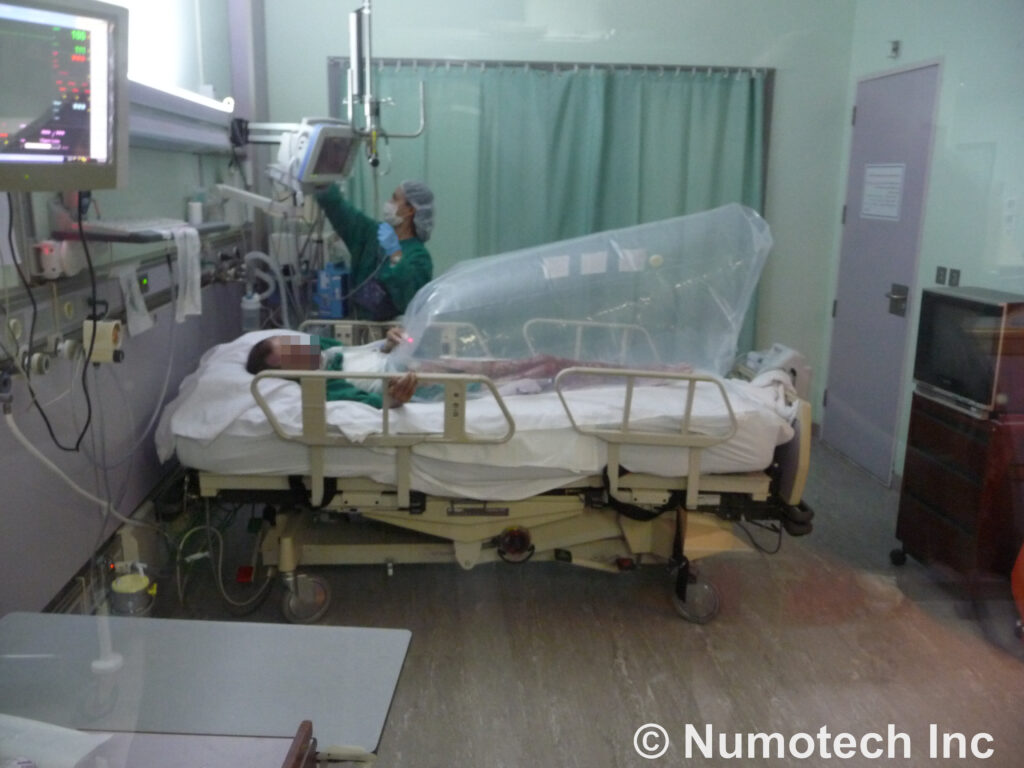
The Numobag® Kit has been battlefield–tested successfully in Iraq and Afghanistan and many other environments. The treatment photos on this site graphically illustrate progressive wound healing of massive advanced-stage wounds, with results of one of many peer-reviewed study conducted at one of the Los Angeles VA Medical Centers, at UCLA, and at private hospitals.
The Numobag® Kit’s healing rate reduces the need for amputations, a life-changing quality of life improvement for at-risk patients.
About Numotech
- A California-headquartered medical devices company formed by a team of University of California clinical scientists at University of California in 1990 that developed a product line of 5 next-generation wound care products. They are planned for launch over the next 36 months into three rapidly growing healthcare sectors: the $50 billion chronic wound care market; the $6 billion back, seating and mobility markets; and the $3 billion oxygen therapy market. Numotech specializes in the research, design, manufacture, and marketing of innovative wound care treatment and prevention products for advanced-stage wounds. We also manufacture assistive medical devices for wheelchair-bound patients.
- Serves one of the fastest-growing components of total healthcare spending globally. Wound care expenditure growth from diabetic wounds and the chronic wounds due to a growing and longer-living elderly population is considered unsustainable and a major concern to healthcare professionals.
Product Portfolio
The company has developed a suite of five products. Three of them are designed primarily to assist in the healing and prevention of wounds—a common and often serious ailment associated with the large and growing population of the wheelchair bound, bed-ridden and diabetic patients. The other two are in the final stage of development: an oxygen generator which creates pure oxygen from the atmosphere and a “universal leg” to cost effectively assist lower limb amputees in developing countries.
1. Numobag® Kit
Numobag® (currently in the market) is a patented Topical Hyperbaric Oxygen Therapy (“THOT®”) chamber that has pre-market FDA cleared as a Regulatory Class II device. It is clinically validated to heal treatment-resistant wound conditions. The Numobag® is reimbursable by New York Medicaid and many third-party payers.
Essentially a portable, disposable plastic chamber, the Numobag® is typically attached at the patient’s chest and applies oxygen at a constant low pressure to the patient’s skin from the chest down. Clinical studies and extended Beta testing in both civilian and military environments by the Company have verified substantial efficacy in promoting the healing of 55 wound conditions, including pressure and diabetic ulcers, necrotizing fasciitis and other diseases characterized by open wounds, as well as many types of burns. The Numobag® Kit can also be used to treat wounds resulting from bioterrorism, chemical and nuclear. A new federal government purchase supply contract to sell product to federal agencies is being negotiated and it is also approved by U.S. Department of Homeland Security for use as a first responder tool.
Wound treatments last approximately 4 hours each and range from 8 to 32 treatments per patient, depending on case severity. The product’s efficacy, relatively low cost and disposability (essential to prevent infection) are compelling purchasing incentives, especially given the absence of proven alternative treatment options. Required both 510(k) and PMA approvals.
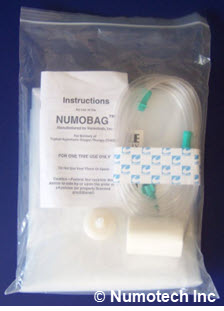
Technology Requirements/Capabilities/Objectives
- Each Numobag® Kit comes complete with all required connectors, tubing, and tape
- Each Numobag® Kit is disposed after one use
- Portable: 1.7 pounds
- Quick Installation: 10-15 minutes to put on, 3-minute removal
- Simple, rapid, non-technical installation
2. Generic Total Contact Seat (GTCS)
Generic Total Contact Seat (GTCS) is a highly user-friendly and portable seat that fits in any wheelchair and promotes local blood flow, periodically relieves pressure, and massages tissues. The biomechanical design systematically compresses tissues, increasing blood supply and stimulating tissue growth. The motorized, battery-powered system is based on high tech weapon systems technology.
The GTCS is the only dynamic wheelchair cushion cleared as a Regulatory Class II medical device by the FDA; and it is the only seat clinically verified to help heal pressure ulcers in the upright position. The upright healing position rapidly improves patients’ quality of life and outlook. The patented GTCS promotes wound healing, cuts treatment time by one-third, and greatly reduces the likelihood of developing new sores by eliminating potential pressure spots.
Two randomized clinical studies favorably comparing a therapeutic high tech seat to a low air loss bed have been completed and the results were published in a highly regarded, peer-reviewed medical journal, Archives of Physical Medicine and Rehabilitation. The GTCS also earned Popular Science magazine’s 2001 “Best of What’s New” award for medical technology.
The GTCS’s competitive pricing, clinically proven efficacy, ability to reduce medical costs, and exclusive Regulatory Class II status are extremely powerful selling points.
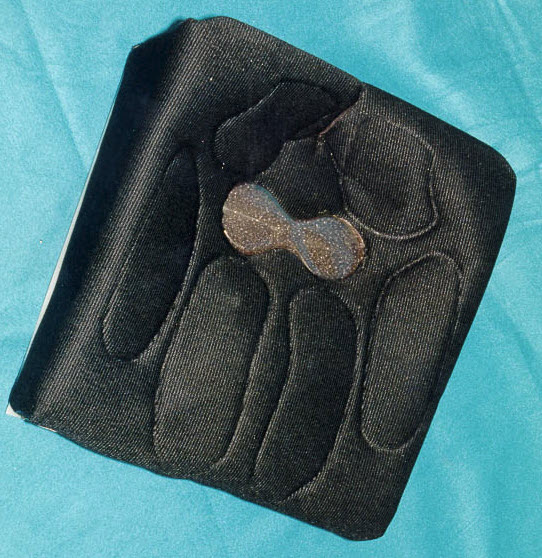
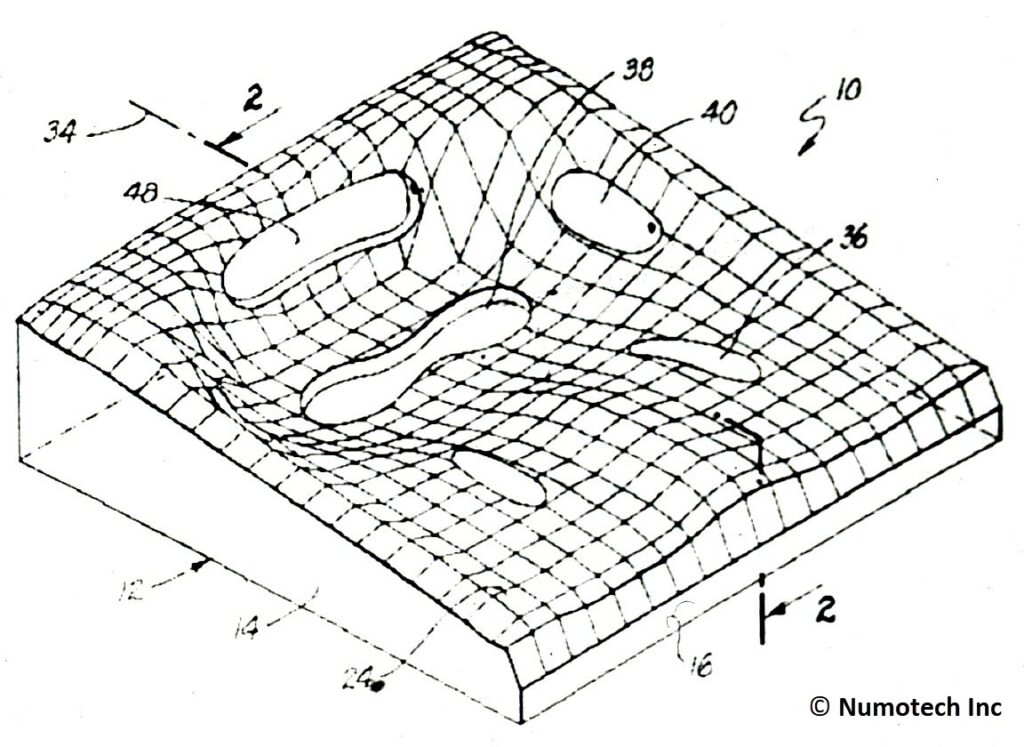
Technology Requirements/Capabilities/Objectives
- Provides an ergonomic fit which does not apply intra-disk
- Uses prosthetic fitting technique analogous to a below-the-knee prosthesis
- Low friction, low shear surface
- Seat cushion with air bladders
- Individually sized to each patient’s anatomy
- Redistributes weight to anatomical areas that can better handle pressure
- Inflating and deflating bladders allow blood circulation to all skin areas by shifting pressure points
- Air compression bladders in the trochanter, ischial, and thigh areas promote blood flow in high pressure skin areas
- Bladders inflate and deflate using a patented sequencing valve arrangement
- Decrease absolute pressure in seating area
- Support bony prominences
3. Back Support System (BSS)

Back Support System (BSS) is a complementary product to the Generic Total Contact Seat (GTCS) that builds on the technologies used in the GTCS. The BSS is FDA-approved and clinically validated to improve patient comfort, stimulate back tissues, and reduce fatigue in wheelchair bound patients.
The BSS is placed behind the patient’s back, allowing the user to vary the level of support in the lumbar area using hand controls to operate a battery-powered pump to pressurize the independently inflatable bladders. This patented system helps to enforce proper posture, support and weight distribution. It is the only product that assists back muscles to provide alignment of the spine while sitting.
The BSS is bio-mechanically designed to provide maximum full-day comfort and relieve back fatigue suffered by wheelchair-bound patients. Patients gain a psychological boost from the ability to independently control their back-rest positioning.
Technology Requirements/Capabilities/Objectives
- Ergonomic fit which does not apply intra-disk pressure in the spinal disks
- Allow variation for back support angle with the seat
- Supports the soft tissues and skeletal structures without direct contact with the spine
- Reduces back pain in wheelchair users due to
- Constant sitting
- Spinal cord injury
- Inability to move independently
- Alleviates continual pounding in wheelchairs without suspension
4. Oxygen Generator (O2 Generator)
Oxygen Generator (O2 Generator) (expected market launch in 2026) is a complementary product to the Numobag® Kit. The O2 Generator is the world’s only lightweight, portable, energy-efficient device capable of producing over 98% pure oxygen from the atmosphere and delivering it at a 15 liters/minute flow rate.
Developed from Sandia National Laboratories patented technology, the O2 Generator’s patented technology will deliver pure oxygen that is a safe and convenient alternative to flammable, heavy and bulky oxygen H-tanks. This non-flammable pure oxygen supply source will enable us to expand the market for Numobag® Kit treatment opportunities to nursing home and in-home settings.
The oxygen source can be used by patients suffering from chronic pulmonary artery disease. There also are significant industrial uses for the O2 Generator. It will be manufactured from durable components and will have a patient-friendly design and lightweight maneuverability.
The O2 Generator is scalable which opens opportunities for deployment to supply oxygen to 300-bed hospitals, as stand-alone oxygen generating systems for rural hospitals, in veterinary medicine, on the battlefield, on aircraft and other non-medical environments where a portable pure oxygen supply is needed
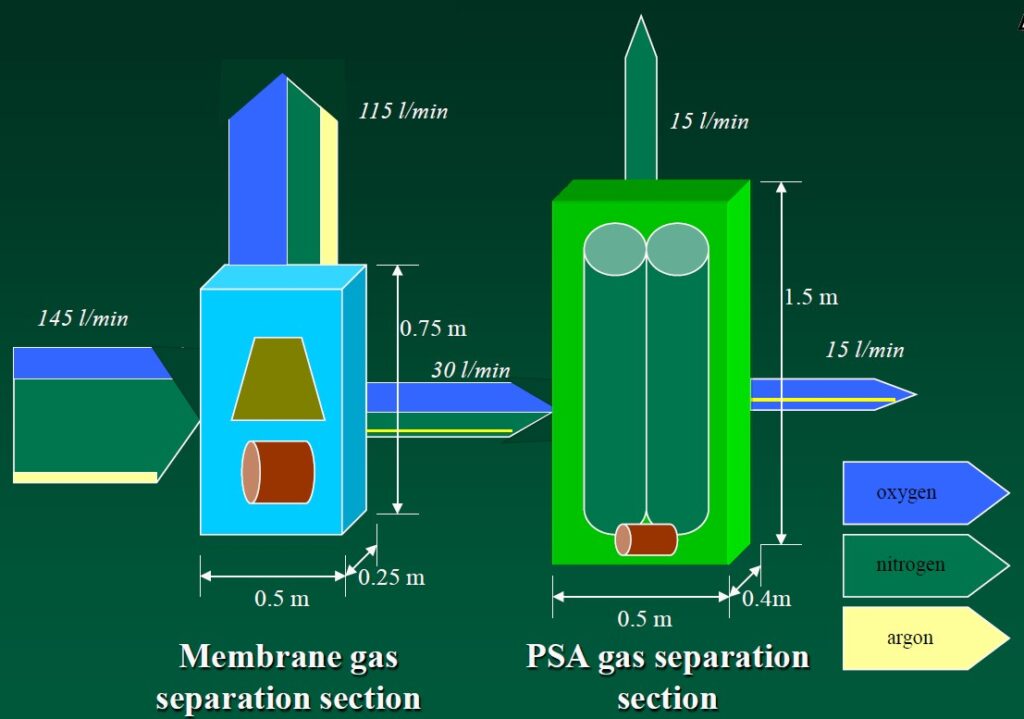
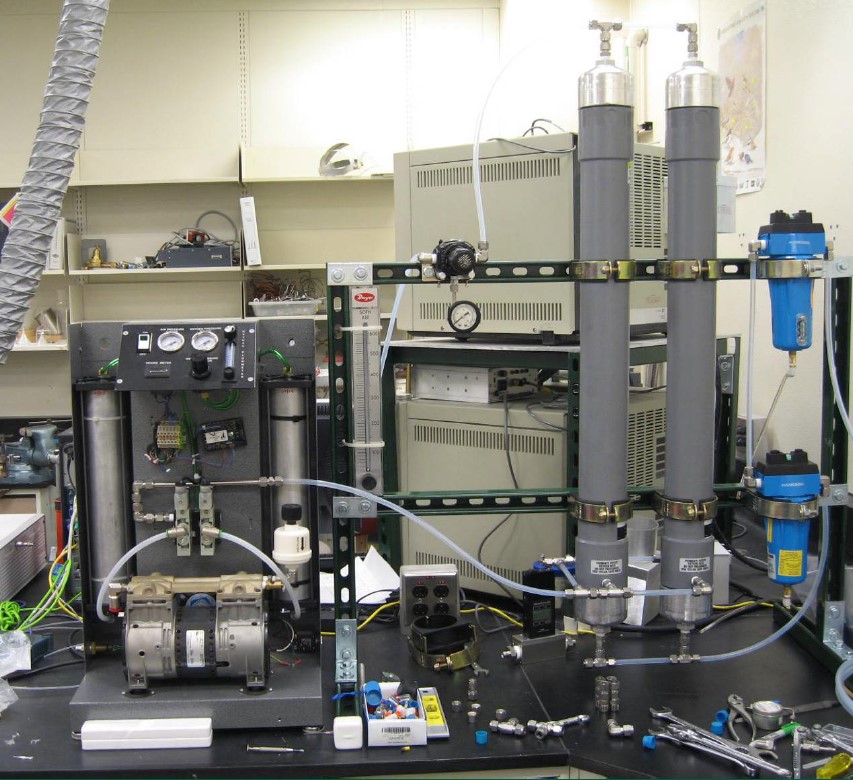
Technology Requirements/Capabilities/Objectives
- Provides at least 15 liters/minute of oxygen
- Flow rate at purity levels greater than 99% oxygen
- Creates 99% oxygen from atmosphere, eliminating bulky, labor intensive & expensive refillable oxygen cylinders
- Constant pressure ranging from 2100 to 2.5 mm H2O
- Regulates delivery pressure to within +/- 2% of desired flow at all flow rates, ranging from 1 to 15 liters per minute
- Produces maximum flow within 5 minutes of start
- Draws less than 1500 watts for maximum/concentrated flow
- Maximum operating temperature of less than 150 degrees Celsius, with no exposed component operating at a temperature of greater than 40 degrees Celsius
- Powered in both US/European without conversion or signal conditioning
- Long-lasting components, user-friendly design, and lightweight portability
5. Universal Leg
The Universal Leg (expected market launch in 2026) will be the world’s first low-cost, high-tech lower leg prosthesis. The product’s relatively low cost will be much less than competing high tech prosthetic devices. Several sizes will be available to ensure proper fit for children or adults and the product will be size adjustable to accommodate their changing needs. This product will increase mobility and other quality of life factors for many people in poor countries. The targeted customer base will include governmental, humanitarian, and health care entities wishing to relieve the burden of amputees in developing countries only.
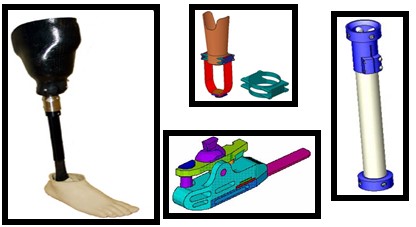
Technology Requirements/Capabilities/Objectives
- Inexpensive
- Maintenance free in a harsh local work and living environment
- Allow user to fit and make periodic adjustments
- Above the knee and below the knee models
- Co-polymer filled socket
- Telescoping pylon
- Adjustable foot/ankle
- Sandia National Laboratories (Albuquerque, NM) is our research partner and adds to the team some of the nation’s most talented technology research scientists and premier engineering laboratories through which to continue to advance the company’s wound care research. We have leveraged U.S. Department of Energy medical innovation research grants and pending funding commitments for additional research. The leveraging strategy adds significant enterprise value at no cost or dilution to investors.
Research Partner: Sandia National Laboratories
Sandia National Laboratories (Albuquerque, NM) is our research partner and adds to the team some of the nation’s most talented technology research scientists and premier engineering laboratories through which to continue to advance the company’s wound care research. We have leveraged U.S. Department of Energy medical innovation research grants and pending funding commitments for additional research.

